
The 2024 Pathbreakers Program brings together the scientific expertise of Novo Nordisk, via its Bio Innovation Hub, and Portal Innovations to work with US-based biotech startups from pre-seed stage onward that are interested in advancing their work through collaboration. The Pathbreakers Program includes different engagement opportunities for consideration depending on a startup’s stage and research collaboration proposal:
- Portal Innovations Ecosystem Placement: Sponsored by the Novo Nordisk Bio Innovation Hub, receive up to one year placement within one of Portal Innovations’ lab and office spaces in Atlanta, Boston, Houston, or Chicago as well as mentorship from a Novo Nordisk scientist.
- Portal Innovations Venture Capital Investment: Portal Innovations may provide venture investment to applicants that meet requirements and scientific scrutiny independently driven from all Novo Nordisk evaluations pertaining to this call.
- Partnership with Novo Nordisk: Depending on the startup’s development stage, the soundness and novelty of its value proposition and the technical/strategic fit with Novo Nordisk, it is possible to be considered for a partnership via Novo Nordisk’s Bio Innovation Hub.
Novo Nordisk’s Bio Innovation Hub is most interested in exploring co-creation opportunities with startups that are developing platform technologies with disease agnostic potential or are interested in exploring scientific projects within the cardiometabolic space. Biotechs that have not previously worked within cardiometabolic diseases but are interested in exploring new indications for their technology or platform are encouraged to apply. Further details on the different engagements available and the focus areas can be found in the “Application Criteria” section.
Application window is closed.
Application Process Opens: December 11, 2023
Virtual Q&A Information Sessions: January 17, 2024 at 1 p.m. ET – Register here.
Application Deadline: January 24th, 2024 by 11:59 pm ET
Evaluation: Evaluation will be done on a rolling basis, so applicants are encouraged to apply earlier than the deadline.
Applicants Notified: Applicants will be notified of selection no later than March 18, 2024.
The Bio Innovation Hub at Novo Nordisk, based in Cambridge, MA, is a cross-disciplinary R&D unit designed to accelerate therapeutic progress and to pioneer a better future in human health through co-creative partnerships. Our focus is on cardiometabolic diseases, with additional interest in rare blood and rare endocrine diseases. We embrace an agile approach to both the science and business of medical innovation as we develop transformative therapeutic solutions.
Portal Innovations is a premier venture development engine with a presence in Atlanta, Boston, Chicago, and Houston, that bridges scientific ideation in life sciences through commercial proof of concept by delivering Crafted Capital, including seed funding, specialized equipment, lab space, and management expertise to high-potential early-stage companies. For more information about Portal Innovations and its portfolio companies, please visit https://www.portalinnovations.com.
What Novo Nordisk is looking for
At Novo Nordisk we believe that disruptive solutions can come from co-creative partnerships where the encounter of ideas and talents from life science innovators finds synergistic convergence with the profound disease understanding, technical and execution capabilities of a leading pharmaceutical company like Novo Nordisk.
To be considered, it is NOT required for the applicant to have already undertaken active research or applications in Novo Nordisk’s therapeutic areas of interest. We prompt the applicants to provide concrete themes of mutual interest that could be tackled in a joint co-creative collaboration. We wish to receive a prospective plan on how an applicant’s work could impact therapeutic or technology areas detailed out below, which clearly highlights strengths, competencies as well as possible Novo Nordisk contributions. In all, this should clearly link to the type of support sought for consideration.
Therapeutic Areas
Novo Nordisk’s purpose is to drive change to defeat serious chronic diseases, built upon our heritage in diabetes. Our current therapeutic focus comprises diabetes, obesity, cardiovascular disease (CVD), Metabolic dysfunction-associated steatohepatitis (MASH, formerly known as non-alcoholic steatohepatitis, NASH), chronic kidney disease (CDK), rare blood disorders (including haemophilia and sickle cell disease) and rare endocrine disorders. All applicants are prompted to suggest a therapeutic connection most closely aligned with their proposal.
It is considered an advantage, but not a prerequisite, if the applicant can demonstrate or suggest approaches and solutions that could directly impact disease as follows:
- Obesity: Reducing appetite & hedonic feeding, increasing energy expenditure, restore impaired endocrine axes while maintaining lean mass during weight loss.
- Diabetes: Preserving β-cell function, restoring insulin sensitivity, improving glucose homeostasis with no risk of hypoglycemia and weight gain.
- CVD: Reducing or preventing the development of heart failure, coronary heart disease, cerebrovascular disease, peripheral artery disease and dyslipidemia.
- MASH: Improving liver health and function for steatohepatitis, in particular by reversing fibrosis and inducing liver regeneration.
- CKD: Improving kidney function in patients suffering from obesity and/or diabetes by halting fibrosis progression and reducing oxidative stress.
Technology Areas
Through the 2024 Pathbreaker Program, we also seek to identify startups that have or are developing cutting edge technologies with disease agnostic potential that can contribute to identifying novel drug targets, drug modalities and delivery solutions.
Moreover, particular consideration would be given to proposals that can enable advancements in:
- Long-acting, scalable multitarget drug modalities to address known biology pathways and mode-of actions
- Approaches or modalities for gain of function and/or upregulation of intracellular targets
- Drug delivery, in particular if conducive to preferential targeting towards specific organs or cell types which are suitable for peptides, proteins, DNA/RNA and gene therapy
- Ability to access certain intracellular space and to control subcellular routing of peptides, proteins, and oligonucleotide therapeutics and gene editing tools
What Portal Innovations is looking for
Portal Innovations’ Crafted Capital approach is specifically designed to meet the needs of emerging life sciences in geographic areas where innovation thrives at the local universities, but the ecosystem lacks the infrastructure to be able to attract VC groups to fund local startups. Early-stage biotech companies developing therapeutics, diagnostics, or medical devices operating in or seriously looking to operate in the Atlanta, Boston, Chicago, or Houston ecosystems can apply.
Portal Innovations remains most interested in, but will consider interest beyond:
- Asset-focused therapeutic companies with strong in vivo data, to fund hit-to-lead and IND-enabling studies.
- Platform-focused therapeutic companies with disruptive technology, to validate and expand platforms and nominate lead assets.
- Diagnostic and medical device companies addressing large unmet medical needs and markets with strong in vivo data, to fund design, manufacturing, and clinical testing.
Overview of types of support available
Biotech startups looking to advance their work through the 2024 Novo Nordisk Bio Innovation Hub and Portal Innovations Pathbreakers Program have multiple potential engagement opportunities, dependent on their development stage, alignment with selection criteria and proposed collaboration plan. The following is a general guide to the types of support available by stage of company funding. When applying, applicants will be asked to clearly articulate the type of support they are seeking, along with a link to their collaboration proposal.
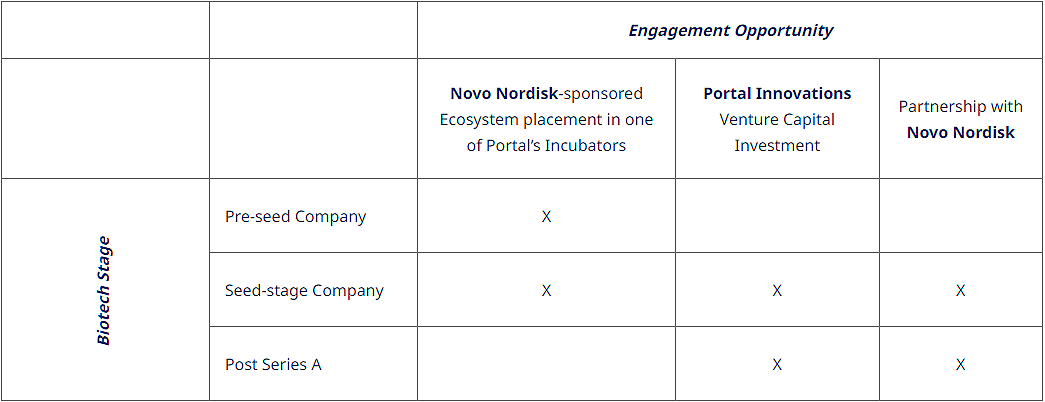
Novo Nordisk Sponsored Portal Innovations Ecosystem Placement
For startups interested in joining one of the ecosystems at Portal Innovations’ Atlanta, Boston, Chicago, or Houston locations, the 2024 Pathbreakers program offers sponsored support from Novo Nordisk’s Bio Innovation Hub for up to $50,000 per startup in lab and office rental fees from Portal Innovations. This also includes the possibility of mentorship and connectivity with a Novo Nordisk Scientist. The opportunity is ideal for startups looking for ongoing support and engagement in the growing ecosystems around Portal Innovations. Startups currently working in other cities but looking to expand with full-time staff into Atlanta, Boston, Chicago, or Houston, are also eligible to apply for this support in addition to those which may already be located regionally to a Portal Innovations location.
Portal Innovations Venture Investment
Portal Innovations may provide venture investment to applicants that meet requirements and scientific scrutiny independently driven from all Novo Nordisk evaluations pertaining to this call. While seed rounds are preferred, companies seeking pre-seed through series A funding will be considered. The venture investment is independent of and may or may not coincide with other engagements offered by Novo Nordisk.
Partnership with Novo Nordisk
Depending on the applicant startup’s development stage, the soundness and novelty of its value proposition and the technical / strategic fit with Novo Nordisk, it is possible to be considered for a partnership via Novo Nordisk’s Bio Innovation Hub. Such a partnership might be in the form of:
- Sponsored Research Agreement (SRA)
A highly focused research collaboration with Novo Nordisk with the goal of the exploration of an ambitious research theme aimed to provide early validation in or build confidence on new biological and/or technological paradigms of strategic importance to Novo Nordisk research activities. Typically spanning over 12 months.
- Collaboration and License Agreement (CLA)
A fully structured and agreed upon partnership, comprising several stages and potentially spanning a broad timeline, e.g. from early research to clinical studies, which is tightly regulated and milestone driven, with payments associated with downstream commercial economics.
At the discretion of the organizers in the review process, other areas of potential support may or may not be considered before the final stages of the program. This call is restricted to startup company applicants operating in and formally registered in the territory of the Unites States of America. Additionally, only companies with at least one full time employee will be considered. To be eligible, the applicants should demonstrate access to the necessary resources (e.g., know-how, lab space, hardware and software) to drive, implement and progress the proposed research theme. See Terms and Conditions for further details.
1. The Program and its General Terms
Novo Nordisk is a global healthcare company with more than 100 years of innovation and leadership in diabetes care.
Novo Nordisk A/S, Denmark ("Novo Nordisk") is hosting a program (the “Pathbreakers Program”) in conjunction with Portal Innovations, LLC (“Portal Innovations”), in which participating entities are eligible to receive various types of awards based on criteria including therapeutic solutions proposed, company stage and position, and expertise and company focus. Novo Nordisk invites interested life-sciences and biotech start-ups to submit proposals for the Pathbreakers Program. The Pathbreakers Program is open for participation by incorporated entities or limited liability companies (each referred to as a “Participant”).
Awards for the Pathbreakers Program (“Awards”) may include:
- A “Portal Innovations Ecosystem Placement” award:
o Winning Participants are eligible to receive sponsored support from Novo Nordisk for up to $50,000 in eligible lab and office rental fees for up to a one-year placement with Portal Innovations ecosystems in Atlanta, Boston, Chicago or Houston, with the possibility of an expanded relationship with a Novo Nordisk scientist.
- A “Partnership with Novo Nordisk” award:
o Winning Participants, depending on the participant’s development stage, the soundness and novelty of their value proposition and the technical and strategic fit with Novo Nordisk, may be eligible for partnerships in the form of a Sponsored Research Agreement or a Collaboration and License Agreement, consistent with Novo Nordisk’s past practices.
- A “Portal Innovations Venture Capital Investment” award, solely awarded by Portal Innovations (and not Novo Nordisk), under and subject to any terms and conditions set forth by Portal Innovations.
These Terms and Conditions between Novo Nordisk and Participants (“Terms”) are applicable to the Pathbreakers Program.
The Entry for the Pathbreakers Program begins on 9:00 AM Eastern Standard Time (EST) on December 11, 2023 and ends on 11:59 PM EST on January 24, 2024, unless terminated earlier by Novo Nordisk (the "Open Call Period").
This initiative is aimed at improving treatment options for people around the world, and includes, but is not limited to, a focus on obesity, diabetes, cardiovascular disease, metabolic dysfunction-associated steatohepatitis (formerly known as nonalcoholic steatohepatitis) and chronic kidney disease.
Note that only duly incorporated or organized legal entities with a US federal tax identification number will be eligible to win an Award.
During the Open Call Period, Participants can submit their solution (“Entry”) via the Pathbreakers Program website.
By submitting an Entry, Participant accepts and agrees to comply with these Terms and with the decisions of Novo Nordisk, which will be final and binding in all respects, including Novo Nordisk’s right to verify eligibility, to interpret, amend or otherwise alter these Terms, and to resolve any claims or disputes relating to the Pathbreakers Program at any time, in any manner, in its sole discretion.
In these Terms, “Novo Nordisk” shall be deemed to include Novo Nordisk A/S, its affiliates, subsidiaries, successors and assigns (not including, in any circumstances, Novo Holdings A/S, Novozymes A/S, NNIT A/S, NNE Pharmaplan A/S or the Novo Nordisk Foundation).
2. Eligibility
Each Participant must be eligible to participate in the Pathbreakers Program and comply with the Terms, or such Participant may be disqualified. The Pathbreakers Program is void in all countries where prohibited by law.
Not eligible to participate in the Pathbreakers Program are:
- companies that do not have at least one full-time employee;
- employees, interns, contractors, representatives, agents and official office-holders of Novo Nordisk, as well as Novo Nordisk’s subsidiaries, affiliates and their respective directors, officers, employees, interns, and contractors, and the immediate family members (such as parents, siblings, children, spouses, life or domestic partners);
- in the U.S.: patients participating in any U.S. government, state, or federally funded medical or prescription benefit programs, including Medicare, Medicaid, Medigap, VA, DOD, and TRICARE. This includes patients participating in a Managed Medicaid plan or who have Medicaid as secondary insurance.
Employees or other affiliates of a Participant who are health care professionals (HCPs) may be required to sign a separate agreement with Novo Nordisk to ensure compliance with Novo Nordisk policies regarding interaction with HCPs. Novo Nordisk will report any transfer of value to an HCP in accordance with U.S. Local/State/Federal laws. Additionally, any transfer of value may be subject to limitations by Local/State regulations.
Participant represents and warrants that Participant’s Entry and actions conducted pursuant to the Pathbreakers Program and ability to comply with these Terms do not violate the Participant’s contractual obligations, policies and procedures.
Novo Nordisk reserves the right to verify eligibility and to adjudicate on any dispute at any time.
To be eligible to win an Award, the winner must be a duly incorporated or organized legal entity with a US federal tax identification number.
3. Phases of the Pathbreakers Program
3.1. Entry Submission
By submitting an Entry, the Participant accepts these Terms.
Entries must be submitted via the Program Website and must include all information required in the online form, including a contact name, email, phone number, company name, company location, funding status and team size. Entries must include confirmation that the Participant has read and understood the terms and conditions, and may be submitted at any time during the Open Call Period.
Additionally, Entries must further include and attach a non-confidential white paper. Such non-confidential white paper must include:
- The Participant’s company background, mission, focus, key areas of research interest, concept or company focus and how it applies to any particular therapeutic area;
- Description of a high-level project plan pertinent for the suggested partnership model. The description should be clear and well-articulated why a given partnership model is preferred and the applicant should be able to suggest timeline and key milestones, and main challenges that remain to be solved. Such description should further identify if there is any existing data that support the idea/concept, and what intellectual property rights exist;
- Participants may put forward more than one suggested partnership model, if applicable, but all Entries must articulate which Award would best-suit the Participant’s stage and proposed project idea;
- Description of the Participant, including the number of employees, funding status (including total amount of funding received at the time of the application), address, phone number and email address as well as a description of resources currently available to Participant (e.g., know-how, lab space, hardware and software) to drive, implement and progress the proposed partnership model; and
- Any additional relevant, non-confidential supporting information (e.g., non-conference slide deck, publication, patents, posters).
An Entry is not complete unless and until the Entry criteria above are properly followed. Submitted entries will not be returned.
Entries will be considered non-confidential. By submitting an Entry, Participant acknowledges and agrees that the Entry will not be treated as confidential. Participant is advised, before submitting an Entry, to consult a lawyer or patent attorney as to the desirability of seeking patents or other protection for the Entry.
By submitting an Entry, Participant warrants and represents that:
i. The Entry is the original work of Participant;
ii. Participant has consented to the submission and use of the Entry in the Pathbreakers Program;
iii. The Entry does not contain any copyrighted material not owned by Participant to the best of Participant’s knowledge, does not infringe the rights of any third party, including but not limited to rights of publicity or privacy, moral rights, or any other property rights;
iv. Participant has the right to present the Entry and to participate in the Pathbreakers Program and there are no claims, judgments or settlements against or owed by Participant relating to the Entry or any information contained therein;
v. The Entry only contains names/likeness/identifying elements of persons for the use of which these persons have given their prior written consent to Participant.
In the Entry white paper, if applicable, Participant must disclose whether:
i. Participant has any currently active formal partnerships with other pharmaceutical companies;
ii. Participant has any current or former relationships (contractual or otherwise) with Novo Nordisk;
iii. Participant employs a Health Care Professional (HCP).
Participant’s submission of the Entry is at its own responsibility and risk. Novo Nordisk shall not be liable for loss of data or illegal intrusion into its systems by third parties.
If a Participant is of the opinion that their solution cannot be further discussed or developed without the disclosure of any confidential or proprietary information or upon the request of Novo Nordisk, Novo Nordisk and any Participant may elect to enter into a confidentiality agreement to ensure that the mutual interests are well protected.
3.2 Review of Entries
The Entries will be judged and scored by a review panel chosen by Novo Nordisk (“Review Panel”) on a rolling basis.
The Review Panel will review the entries for compatibility using criteria including:
1. Fit to Novo Nordisk R&D plan and ambitions, in keeping with the Therapeutic Areas and Technology Areas statements present in the call website and the development stage of the Participant.
2. Project plan. To which extent can the hypothesis/idea/concept be experimentally tested and is compatible with the proposed partnership model.
3. Innovation height. To which extent does the idea/concept differ from existing drugs/solutions of public domain or on the market?
Novo Nordisk reserves the right to assess the Entries in its sole discretion, which may include interviews or discussions with certain Participants.
3.3 Selection of Participants
Novo Nordisk may, through its Review Panel, independently select any number of submitted Entries for interviews (referred to as “Selected Participants”), subject to verification and compliance with these Terms. The format and number of interviews may vary depending on the partnership model and the scientific nature of the Entry.
If an email notification is returned as undeliverable, or if Selected Participants do not respond within the required number of days specified by Novo Nordisk, or if any information submitted by Participant is found in non-compliance with the Terms, raises concern to Novo Nordisk, or if potential Selected Participants decide to decline selection as Selected Participants for any reason, Novo Nordisk shall have no further obligations to such Selected Participants and a place as Selected Participants may instead be awarded to a runner-up Selected Participant, time-permitting and at Novo Nordisk’s sole discretion.
The Selected Participants may receive questions or other feedback from Novo Nordisk after being selected as Selected Participants.
3.4. Selection of Winners
No later than March 18, 2024, Novo Nordisk will communicate with the Selected Participants via the email address they provided if (i) such Selected Participant will be receiving an Award (a “Winner”), (ii) there is still interest in deepening scientific or partnership discussions with such Selected Participant and/or there is a request for additional information or (iii) Novo Nordisk has elected not to award such Selected Participants any Awards. Such selections shall be made at Novo Nordisk’s sole discretion and Novo Nordisk shall, at its sole discretion, determine whether any Selected Participants under clause (ii) will later receive any Awards.
If an email notification is returned as undeliverable, if a Winner does not respond within the required number of days specified by Novo Nordisk, if any information submitted by Participant is found in non-compliance with the Terms or raises concern to Novo Nordisk in Novo Nordisk’s sole discretion, or if a Winner decides to decline the prize for any reason, Novo Nordisk shall have no further obligations to such Winner and the applicable prize will be forfeited and may be awarded to a runner-up Winner, time-permitting and at Novo Nordisk’s sole discretion.
As stated above in “Eligibility”, to be eligible to win any Awards, the Winner must be a duly incorporated or organized legal entity with a US federal tax number.
Any Winners awarded a Portal Innovations Ecosystem Placement Award will, subject to any terms and conditions set forth by Portal Innovations, have up to $50,000 in eligible lab and office rental fees associated with Portal Innovations on terms to be decided by Novo Nordisk following the award of the Portal Innovations Ecosystem Placement Award. Eligibility of such fees will be determined in Novo Nordisk’s sole discretion and Novo Nordisk shall have no obligation to the Winner to enter into any agreement with respect to Portal Innovations Ecosystem Placement Award if the parties cannot mutually agree to applicable terms and conditions.
Any Winners awarded a Partnership with Novo Nordisk Award will, following announcement of the Award, negotiate in good faith with Novo Nordisk the terms for a research agreement proposed by Novo Nordisk. If negotiations are unsuccessful, Novo Nordisk shall have no obligation to the Winner to enter into any research agreement, including any agreement or arrangement proposed by the Winner.
The Awards are non-transferable and substitutions or cash redemptions will not be allowed. Except where prohibited by law, all tax liabilities are the responsibility of the Winner. Novo Nordisk will not be responsible for any tax deductions which may be necessary. Participant acknowledges that it will not be entitled to any additional payment by reason of any award(s) being subject to any tax, levy, or other charge in any jurisdiction.
Winners are responsible for any additional costs and expenses associated with the acceptance and/or use of any Award. All details of the prize not specified in these Terms shall be determined by Novo Nordisk in its sole discretion.
Any Awards granted by Portal Innovations shall be entered into solely between the Winner and Portal Innovations on separate terms between Portal Innovations and the Winner. Under no circumstances will Novo Nordisk be responsible for the provision of any Awards granted by Portal Innovations.
4. Indemnification, warranties and acknowledgements
Participant accepts the conditions stated in these Terms, agrees to be bound by all decisions of Novo Nordisk regarding the Pathbreakers Program, and warrants that Participant is eligible to participate in the Pathbreakers Program as specified in these Terms.
Participant indemnifies Novo Nordisk for any damages (including payment of reasonable attorneys’ fees) in connection with Participant’s participation in the Pathbreakers Program and Participant’s acceptance and use of any Awards.
Participant indemnifies Novo Nordisk for any damages (including payment of reasonable attorneys’ fees) in connection with any claim for misappropriation or infringement resulting from (i) Participant’s infringement of any third party’s intellectual property rights; (ii) Novo Nordisk’s mentoring of Participant; or (iii) Novo Nordisk’s involvement with any idea, invention, information or materials comprised in the Entry.
Participant acknowledges that Novo Nordisk may presently, during the Pathbreakers Program, and/or in the future, be developing internally, or receiving from other parties, ideas, concepts, solutions and information that are similar to the Entry. Accordingly, nothing herein shall prohibit Novo Nordisk from independently acquiring, developing, or having developed for it, products, concepts, systems, services, or techniques that are similar to or compete with the products, concepts, systems or techniques contemplated by or embodied in the Entry.
Participant will not in any manner undermine the integrity of the Pathbreakers Program. Participant will not use any device, software or routine to interfere with the proper working of the Program Website or with the intention to damage, interfere with or surreptitiously intercept or expropriate any system, data or personal information.
5. Other
i. No offer or payment for products or services.
This Pathbreakers Program and the grant of any of the possible Awards are not intended to be, nor shall these be construed as, an offer or payment made, whether directly or indirectly, to purchase, lease or order of any item or service of Novo Nordisk.
ii. Compliance with requirements.
Determination of compliance with Entry, technical, and other requirements and these Terms will be in the sole discretion of Novo Nordisk. Novo Nordisk reserves the right to disqualify any Participant whose participation may, or whose Entry may, cause controversy or negative publicity for the Pathbreakers Program, Novo Nordisk or any third parties. Participant shall not use the Novo Nordisk name, logo, corporate identity or images without Novo Nordisk’s prior written consent.
iii. No liability.
Novo Nordisk assumes no responsibility for the following: any problems, technical malfunctions or delays in electronic operations or transmissions; entries that are lost, stolen, incomplete, damaged, garbled, destroyed, misdirected or not received for any reason; destruction of or unauthorized access to, or alteration of entries or related material, failed or unavailable hardware, network, software or telephone transmissions, damage to Participant’s or any person’s computer and/or its contents related to or resulting from participation in the Pathbreakers Program; or any errors in these Terms or in any advertisements or correspondence in connection with the Pathbreakers Program.
iv. Participant consent.
Participant consents, authorizes and grants to Novo Nordisk the irrevocable and unrestricted right and permission to take, copyright, use and publish printed, video, audio, or photographic images of Participant and Participant’s statements, in whole or in part, in conjunction with or without Participant’s name, or any reproductions thereof related to the Pathbreakers Program for use with internal and external audiences, including the right to edit these materials to ensure compliance with applicable rules and regulations.
vi. Novo Nordisk decisions.
Novo Nordisk’s decisions are final and binding in all matters relating to the Pathbreakers Program.
vii. Precedence.
In the event of any inconsistency between these Terms and any other provisions published or otherwise communicated in relation to the Pathbreakers Program, these Terms shall prevail.
viii. Program cancelation or suspension.
Novo Nordisk reserves the right to cancel or suspend the Pathbreakers Program at any time at its sole discretion.
6. Personal Data & Privacy
Entries will include information relating to identified or identifiable natural persons (“Personal Data”) and, in particular, may include name, title, email address, mailing address, phone number and age of Participant’s employees, advisors or affiliates.
By submitting Personal Data of individuals associated with a Participant, the Participant represents and warrants that all necessary permissions from all individuals associated with the Participant have been obtained.
Novo Nordisk collects, processes, and/or uses Personal Data submitted for the purposes of the Pathbreakers Program in accordance with these Terms, in particular for verifying the identity of Participants, for administering the Pathbreakers Program, and to contact Participants for the organization and execution of the Pathbreakers Program.
Novo Nordisk may anonymize and aggregate data collected through the Pathbreakers Program Website for statistical purposes to help improve its products and services.
Participant expressly consents to: (i) the collection, use and retention by Novo Nordisk of Participant’s personal and business information contained in the Entry for all purposes (including promotion and publicity) related to the Pathbreakers Program and for the purposes set forth more fully on Novo Nordisk’s website, as well as for use in a publicly available Selected Participant and Winners list; and (ii) the publication of Participant’s name, picture and entrepreneurial story on the Program Website, Novo Nordisk websites as well as on Novo Nordisk’s social media channels (Facebook, Twitter, YouTube, Instagram, etc.).
7. Disputes
Participant agrees that any and all disputes, claims and causes of action out of or connected with the Pathbreakers Program shall be resolved individually, without resort to any form of class, mass or other collective action.
All issues and questions with regard to the construction, validity, interpretation and enforceability of these Terms, or the rights and obligations of Participant and Novo Nordisk shall be governed by, and construed in accordance with, the laws of the state of New York, USA, without giving effect to any choice of law or conflict of law rules, which would cause the application of the laws of any other jurisdiction.
The exclusive jurisdiction and venue of any action with respect to the subject matter of these Terms shall be the state courts of the State of New York or the United States District Court for the Southern District of New York; and Participant submits itself to the exclusive jurisdiction and venue of such courts for the purpose of any such action. Any and all claims, judgments, and awards shall be limited to actual out-of-pocket costs incurred, including costs associated with participating in the Pathbreakers Program, but in no event attorneys’ fees. Participant hereby waives all rights to (i) claim or be awarded any punitive, direct, indirect, incidental, and consequential damages and any other damages, other than for actual out-of- pocket expenses, and (ii) to have damages multiplied or otherwise increased, including for willful patent infringement.