Novo Nordisk is its people. We know that life is anything but linear and balancing what is important at different stages of our career is never easy. That is why we make room for diverse career aspirations and always put people first. We value our employees for the unique skills they bring to the table, and we work continuously to bring out the best in them. Working at Novo Nordisk is working toward something bigger than ourselves, and it is a collective effort. Novo Nordisk relies on the joint potential and collaboration of its more than 59,000 employees. Together, we go further. Together, we are life changing.
Read more about Novo Nordisk Quality here .
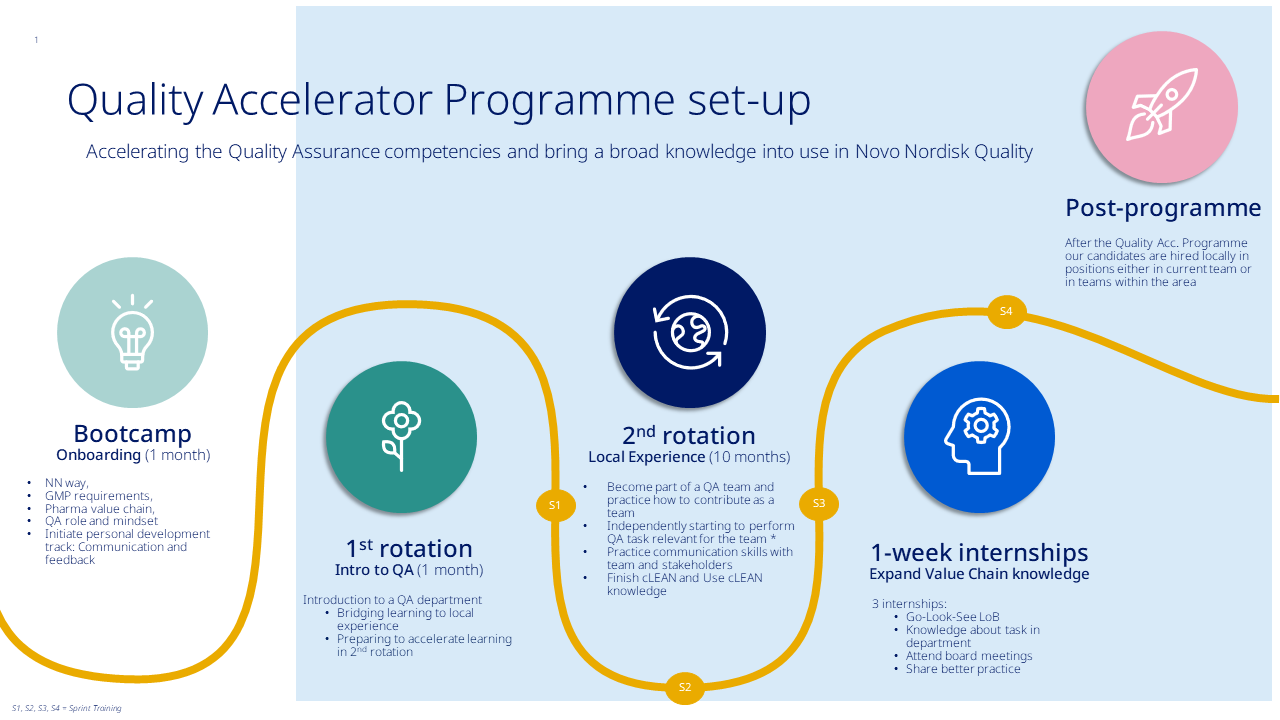
The Quality Accelerator Programme will start 1st of September 2024 and the deadline for applying is the 7th of April 2024.
Please do not attach a cover letter to your application, instead you will have to include a short description about why you are applying for this programme in your resume or CV. Also, you will have to state if you are interested in the Kalundborg location, the Greater Copenhagen incl. Hillerød location or both. If you do not include this, we will not be able to consider your application.
For further information, feel free to contact Programme Lead, Sarah Tang at +45 30 79 53 93 or Programme Lead, Mathilde Hoff at +45 30 75 87 79